Access to Drug-like Candidates
Access to Drug-like Candidates




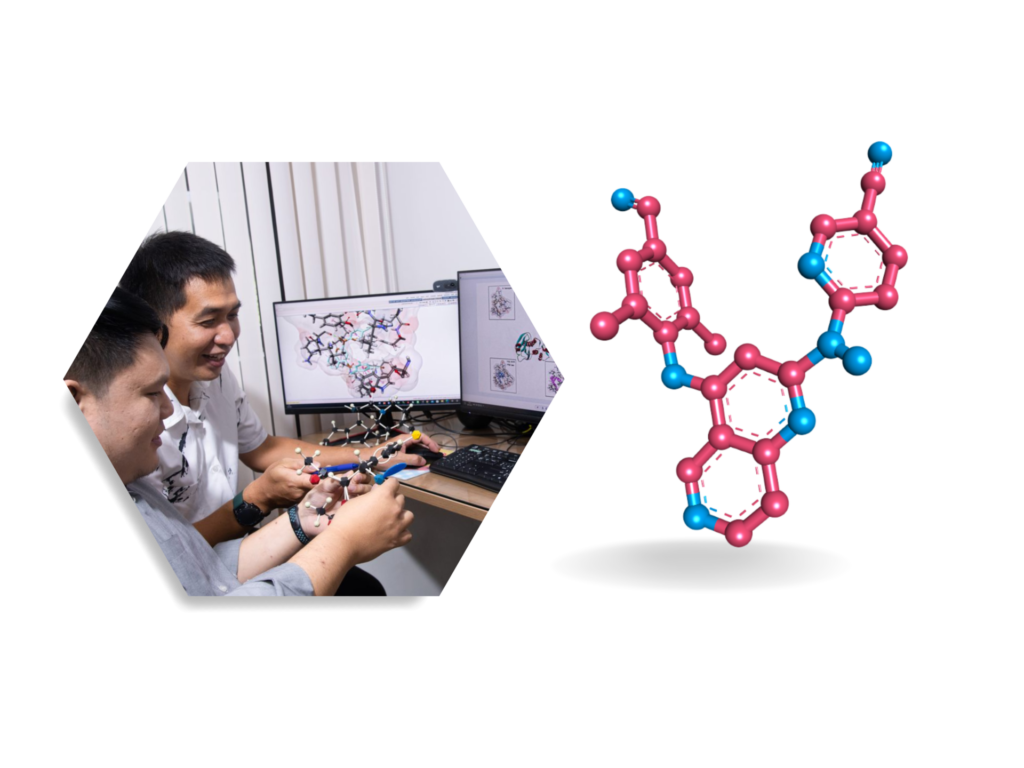
The typical first stage of rational drug design involves the search for molecular templates from which more desirable candidates can be accessed via direct structural modifications or de novo synthesis. On the basis of herbal or folk medicines worldwide, natural products, with their unimaginably complex novel skeletons, have served as both concrete entities and inspiration to learn from as well as to design the better analogs with an aid of modern computational chemistry techniques.
Various clinically used herbal remedies with their known bioactive components have been our focus to establish the fully integrated approach for herbs, featuring the development of simplified and efficient isolation processes suitable for industrial applications. Advanced technologies and instrumentation have enabled the quantitative determination of the natural abundance of major bioactive compounds and the preparation of compound standards for commercialization. In addition, our laboratory has also employed some of these naturally occurring compounds as starting materials for direct derivatizations. As an example, mitragynine, the major alkaloid component in Mitragyna speciosa, locally known as Kratom, has been obtained through this approach and employed to prepare the bioactive metabolite, 7-hydroxymitragynine, along with other semi-synthetic analogs.
As a crucial alternative to access bioactive natural compounds with low natural abundance, simplified and efficient synthetic methods have also been devised for heterocycles frequently encountered as pharmacophores. Structural modifications either on a single platform by a conventional approach or on a combined platform by a modern hybrid approach has been designed to provide new chemical entities with better efficacy. The latter has enabled the combination of two different bioactive components through a linker, yielding a new “chimeric” molecule. Various modern organic reactions, with atom-, oxidation-, and step-economy, have been contemplated and utilized to maximize the reaction efficiency. Some of these synthetic chimeras have shown higher efficacy because of novel synergistic effects upon their interactions with known or new molecular targets.





