Total Synthesis of Natural Products
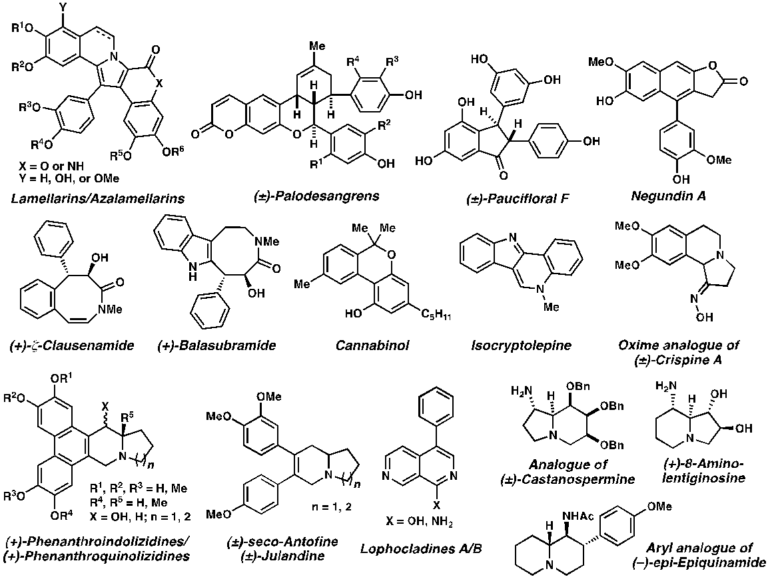
Challenges in total synthesis of natural products lie in the disconnection and execution of series of chemical reactions. Ingenious insights and capability to exploit and manipulate available repertoire of chemical methods as well as subtle differences in chemical reactivities are crucial for the successfully designed synthetic approaches with highest efficiency. In addition, utility of biomimetic/bio-inspired syntheses has also been illustrated. Stereochemical outcomes of each step for those containing stereogenic centers are important considerations upon devising synthetic routes. Divergent synthesis has also played an important role in designing a synthetic strategy to a common intermediate for the preparation of other analogs, thus increasing the efficiency and flexibility of such developed route. State-of-the-art synthetic approaches for various cores of natural products have been designed and executed successfully. Over the years, our laboratory has prepared both plant- and marine-derived natural products and their analogs including: lamellarins/azalamellarins, (±)-palodesangrens, (±)-paucifloral F, negundin A, (+)-ζ-clausenamide, (+)-balasubramide, cannabinol, isocryptolepine, (±)crispine A, (+)-phenanthroindolizidine, (+)-phenanthroquinolizidine, (±)-seco-antofine, (±)-julandine, lophocladines, (±)-castanospermine, (+)-8-aminolentiginosine, and (-)-epi-epiquinamide.